Abstract
Background
Allogeneic (off the shelf) chimeric antigen receptor (CAR) T-cell therapy addresses the logistical challenges, availability (including insufficient T-cell yields from low baseline absolute lymphocyte count), and variable product quality of autologous CAR T therapy. ALLO-715 is a genetically modified anti-BCMA AlloCAR T™ cell which uses Cellectis TALEN ® technology to disrupt the T-cell receptor alpha constant gene (TRAC) and the CD52 gene to reduce the risk of graft-versus-host disease (GvHD) and permit the use of ALLO-647, an anti-CD52 monoclonal antibody (mAb), for selective and transitory host lymphodepletion (LD).
Methods
UNIVERSAL is an open-label, Phase 1 trial (NCT04093596) in adults with relapsed/refractory (R/R) multiple myeloma who have received ≥3 prior lines of therapy including a proteasome inhibitor, immunomodulator, and anti-CD38 mAb. Patients (pts) must be refractory to their last treatment line. Pts receive LD followed by ALLO-715 at 1 of 4 dose levels (DLs) in a 3+3 dose escalation design: 40, 160, 320, and 480 x 10 6 CAR+ T cells. Several LD regimens are being evaluated: FCA39; FCA60; FCA90; and CA39; with fludarabine [F] 90 mg/m 2, cyclophosphamide [C] 900 mg/m 2, and ALLO-647 [A] 39, 60, or 90 mg divided over 3 days.
Results
As of June 21, 2021 data-cut, 47 subjects were enrolled; 42 were treated with ALLO-715; 5 progressed prior to treatment. Median time from enrollment to LD was 5 days and no treated pts required bridging therapy. Pts were heavily pretreated with a range of 3-11 prior lines of therapy; 42.9% were penta refractory. There were 19% ISS Stage III at screening, 34% had high risk cytogenetics, and 19% had extramedullary disease. The most common Grade (Gr) 3+ adverse events (AEs) included anemia, neutropenia, lymphopenia, and thrombocytopenia. Cytokine release syndrome (CRS) occurred in 52.4%, all Gr 1/2 except 1 pt with Gr 3. CRS was treated with tocilizumab (21.3%) and corticosteroids (12.8%). One pt (with Gr 2 CRS) experienced Gr 1 neurotoxicity that resolved. Gr 3+ infections occurred in 12.8% of pts, including 2 previously reported Gr 5 events (fungal pneumonia and adenovirus hepatitis).
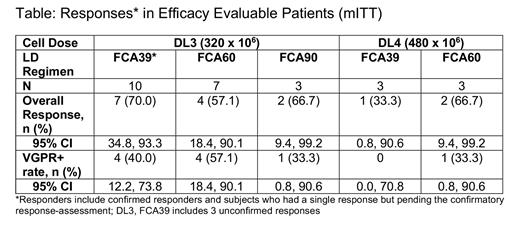
Early results from this trial have been presented previously (ASH 2020) and therefore the efficacy analysis on pts (n=26) treated at DL3 and DL4 with FCA LD, which are considered a more relevant cell dose and LD, is presented here (Table). In pts who received DL3 or DL4 (320 or 480 x 10 6 CAR T cells) (n=26), the ORR was 61.5% (95% CI: 40.6, 79.8); very good partial response or better (VGPR+) rate was 38.5% (95% CI: 20.2, 59.4). Median time to 1 st response was 16 days. The median follow-up for the DL3/DL4 pts was 7.4 months (95% CI: 3.6, 9.9) with a median duration of response of 8.3 months (95% CI: 1.5, not reached). Of the 10 pts with a best response of VGPR+, 8 were found to be negative by minimal residual disease (MRD) analysis.
Conclusions
UNIVERSAL demonstrates proof of concept for allogeneic CAR T therapy in multiple myeloma. ALLO-715 showed CAR T cell dose response relationship with higher doses (DL3/DL4) leading to clinically meaningful efficacy, including high VGPR+ rate of 38.5% and high MRD negativity without requiring leukapheresis or bridging therapy. ALLO-715 following LD with FCA has a tolerable safety profile. FCA induces a deep and durable window of lymphocyte depletion allowing CAR T cell expansion and persistence. A study using the next generation anti-BCMA CAR (ALLO-605) which supplies cytokine signaling to the CAR bearing cells is ongoing (IGNITE). The current UNIVERSAL trial continues to enroll, including a cohort with consolidation, and updated data will be presented at the meeting.
Mailankody: Allogene Therapeutics: Research Funding; Plexus Communications: Honoraria; Fate Therapeutics: Research Funding; Physician Education Resource: Honoraria; Bristol Myers Squibb/Juno: Research Funding; Jansen Oncology: Research Funding; Legend Biotech: Consultancy; Evicore: Consultancy; Takeda Oncology: Research Funding. Liedtke: Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Kura Oncology: Membership on an entity's Board of Directors or advisory committees; Alnylam: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Caelum: Membership on an entity's Board of Directors or advisory committees, Other: Clinical Trial Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Sidana: BMS: Consultancy; Magenta Therapeutics: Consultancy, Research Funding; Allogene: Research Funding; Janssen: Consultancy, Research Funding. Chhabra: GSK: Honoraria. Oluwole: Janssen: Consultancy; Pfizer: Consultancy; Curio Science: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding. Kumar: Oncopeptides: Consultancy; Antengene: Consultancy, Honoraria; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bluebird Bio: Consultancy; Roche-Genentech: Consultancy, Research Funding; Merck: Research Funding; Carsgen: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Research Funding; Beigene: Consultancy; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Tenebio: Research Funding; Sanofi: Research Funding. Nath: Actinium: Consultancy, Honoraria; Incyte: Consultancy, Honoraria. Anwer: Janssen pharmaceutical: Honoraria, Research Funding; Allogene Therapeutics: Research Funding; GlaxoSmithKline: Research Funding; BMS / Celgene: Honoraria, Research Funding. Raje: Caribou: Other; Janssen: Other; bluebird bio: Other; Amgen: Other; Celgene: Other; BMS: Other. Siegel: Bristol Myers Squibb: Honoraria, Speakers Bureau; Takeda: Honoraria; GlaxoSmithKline: Honoraria, Speakers Bureau; Celularity: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Honoraria; Amgen Inc.: Honoraria; Janssen: Honoraria, Speakers Bureau. Karski: Allogene Therapeutics: Current Employment, Current equity holder in publicly-traded company; Crispr Therapeutics: Current equity holder in publicly-traded company; Nektar Therapeutics: Current equity holder in publicly-traded company. Lovelace: Allogene Therapeutics, Inc.: Current Employment. Lourbakos: Allogene Therapeutics, Inc.: Current Employment. Hari: Millenium: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Adaptive Biotech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Karyopharm: Consultancy; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Celgene-BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal